MVA-MERS-S-Phase 1a
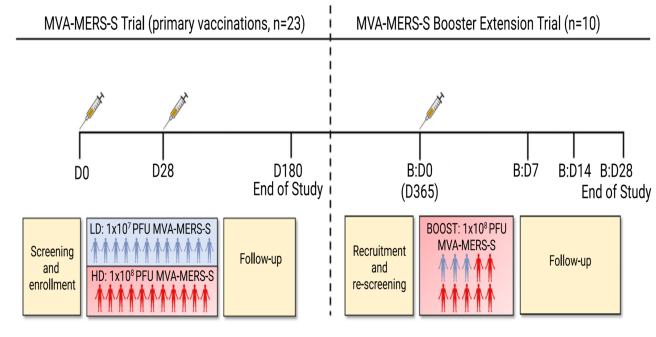
The MVA-MERS-S trial was an open, single center Phase Ia trial to assess the safety, tolerability and immunogenicity of two ascending doses of the candidate vaccine MVA-MERS-S.
The vaccine trial was conducted in collaboration with the Clinical Trial Center North (CTC North). A total of 23 healthy trial volunteers were vaccinated twice with MVA-MERS-S with an interval of four weeks between the vaccinations. The aim of the trial was to answer two questions: Is the vaccine tolerated well and safe to use in humans and does it trigger the development of antibodies and T cells that are able to prevent MERS-CoV infection.
The vaccine candidate MVA-MERS-S is based on an attenuated viral vector (MVA: modified vaccinia virus Ankara) and been altered to contain protein components from the MERS coronavirus. This recombinant, vector-based vaccine is to boost immunity against MERS coronaviruses. Prof. Gerd Sutter from Ludwig-Maximilians University of Munich developed this vaccine in collaboration with Philipps University of Marburg and the Erasmus Medical Center Rotterdam.
The findings of the Phase Ia trial are published in:
Fathi A, Dahlke C, Krahling V, Kupke A, Okba NMA, Raadsen MP, Heidepriem J, Muller MA, Paris G, Lassen S, Kluver M, Volz A, Koch T, Ly ML, Friedrich M, Fux R, Tscherne A, Kalodimou G, Schmiedel S, Corman VM, Hesterkamp T, Drosten C, Loeffler FF, Haagmans BL, Sutter G, Becker S, Addo MM. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat Commun. 2022 Jul 19;13(1):4182. doi: 10.1038/s41467-022-31557-0. PMID: 35853863. Fathi et al
Weskamm LM, Fathi A, Raadsen MP, Mykytyn AZ, Koch T, Spohn M, Friedrich M; MVA-MERS-S Study Group, Haagmans BL, Becker S, Sutter G, Dahlke C, Addo MM. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep Med. 2022 Jul 19;3(7):100685. doi: 10.1016/j.xcrm.2022.100685. PMID: 35858586; PMCID: PMC9295383.
Weskamm et al
Koch T, Dahlke C, Fathi A, Kupke A, Krähling V, Okba NMA, Halwe S, Rohde C, Eickmann M, Volz A, Hesterkamp T, Jambrecina A, Borregaard S, Ly ML, Zinser ME, Bartels E, Poetsch JSH, Neumann R, Fux R, Schmiedel S, Lohse AW, Haagmans BL, Sutter G, Becker S, Addo MM. Safety and immunogenicity of a modified vaccinia virus Ankara vector vaccine candidate for Middle East respiratory syndrome: an open-label, phase 1 trial. Lancet Infect Dis. 2020 Jul;20(7):827-838. doi: 10.1016/S1473-3099(20)30248-6. Koch et al.
New publication on the MVA-MERS-S booster vaccination in Nature Communications
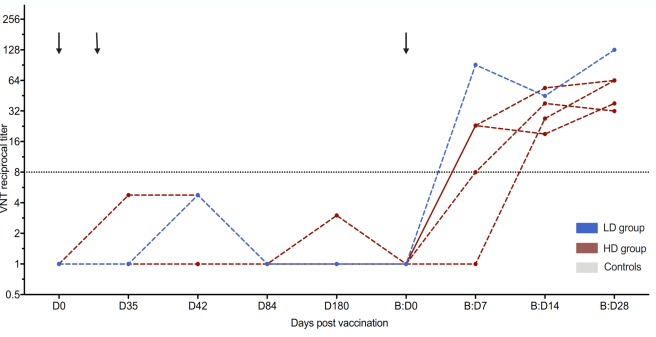
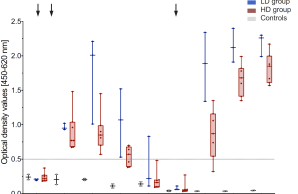
Fathi et al. evaluate the safety and immunogenicity of a third vaccination with MVA-MERS-S in a subgroup of trial participants one year after primary immunization Link to vaccine trial . The results show that MVA-MERS-S booster vaccination is safe and well-tolerated. Both binding and neutralizing anti-MERS-CoV antibody titers increase substantially in all participants and exceed maximum titers observed after primary immunization more than 10-fold.
The data by Fathi et al. support the rationale of a booster vaccination with MVA-MERS-S and encourage further investigation in larger trials.
Fathi A, Dahlke C, Krähling V, Kupke A, Okba NMA, Raadsen MP, Heidepriem J, Müller MA, Paris G, Lassen S, Klüver M, Volz A, Koch T, Ly ML, Friedrich M, Fux R, Tscherne A, Kalodimou G, Schmiedel S, Corman VM, Hesterkamp T, Drosten C, Loeffler FF, Haagmans BL, Sutter G, Becker S, Addo MM. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat Commun. 2022 Jul 19;13(1):4182. doi: 10.1038/s41467-022-31557-0 Fathi et al.
Contact Anahita Fathi, Clinician Scientist
20.07.2022
New publication on the MVA-MERS-S vaccine candidate
Weskamm et al. longitudinally describe B and T cell responses as well as antibody subclasses and neutralization capacity induced by three homologous immunizations with the MVA-MERS-S vaccine candidate.
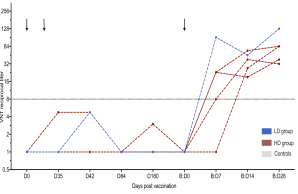
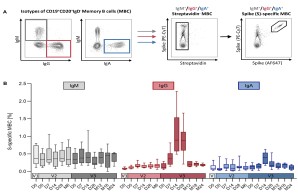
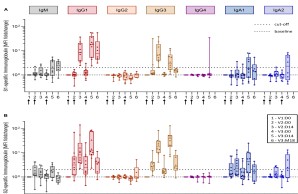
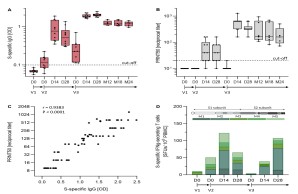
The Middle East Respiratory Syndrome (MERS) is a respiratory disease caused by MERS coronavirus (MERS-CoV). In follow-up to a phase 1 trial, we perform a longitudinal analysis of immune responses following immunization with the Modified Vaccinia virus Ankara (MVA)-based vaccine MVA-MERS-S encoding the MERS-CoV-spike protein. Three homologous immunizations were administered on days 0 and 28 with a late booster vaccination at 12±4 months. Antibody isotypes, subclasses and neutralization capacity as well as T and B cell responses were monitored over a period of three years using standard and bead-based ELISA, PRNT50, ELISpot and flowcytometry. The late booster immunization significantly increases frequency and persistence of spike-specific B cells, binding IgG1 and neutralizing antibodies, but not T cell responses. Our data highlight the potential of a late boost to enhance long-term antibody and B cell immunity against MERS-CoV. Our findings on the MVA-MERS-S vaccine may be of relevance for COVID-19 vaccination strategies.
Weskamm LM, Fathi A, Raadsen MP, Mykytyn AZ, Koch T, Spohn M, Friedrich M, MVA-MERS-S Study Group, Haagmans BL, Becker S, Sutter G, Dahlke C, Addo MM. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep Med. 2022 in press
Contact Marie Weskamm
23.06.2022