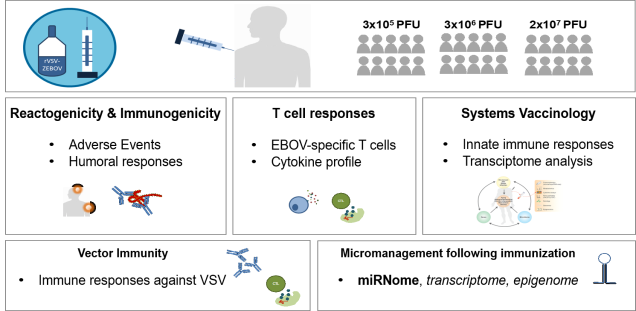
The IIRVD investigates and develops vaccine strategies for infections with emerging viruses such as Ebola virus and coronaviruses (SARS-CoV-2 and MERS) and other clinically relevant viruses (including HIV, HBV).
In several research projects, we analyze the immune responses induced by vaccines. In particular, we are studying early immune events initiated by the innate arm of the immune system, as well as B- and T-cell responses. We are also investigating sex-specific differences in vaccine responses.
Our research is mainly conducted at the Bernhard Nocht Institute for Tropical Medicine in the Department for Clinical Immunology of Infectious Diseases .
Research Projects
Deciphering adaptive immunity in response to vaccines using immune organoids
In response to epidemic or pandemic outbreaks caused by (re-)emerging viruses such as Ebola virus (EBOV), Middle East respiratory syndrome-related coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), rapid vaccine development is imperative. Currently, the study and characterization of vaccine-induced immunity is largely limited to preclinical animal models and ex vivo analyses of human peripheral blood cells from vaccinated individuals. However, animal models often fail to adequately predict human immune responses. Besides, only a fraction of the immune cells necessary for the development of a long-term immune response are found in the blood. Certain populations such as follicular T helper cells, which play a pivotal role in the activation of germinal center B cells, are found almost exclusively in lymphoid tissue.
This gap can be bridged by using human immune organoid technologies as an in vitro model for investigation of adaptive immune responses to infections and vaccine candidates. We are currently establishing a lymphoid tissue organoid model, using tissue that is removed during clinically necessary tonsillotomies and adenotomies. In general organoids offer the potential to complement in vivo experiments involving animal models, while at the same time providing a more accurate prediction of vaccine responses in humans. Especially with regard to respiratory infections, human tonsils are one of the body’s first line of defense. Therefore, investigation of infection and vaccination effects in organoids derived from this tissue is of particular interest. By stimulation of the immune organoids with vaccine candidates against SARS-CoV2, MERS-CoV and EBOV, we are analyzing underlying mechanisms of differential adaptive immunity to these vaccine candidates.
This project is a collaboration between the Department of Otolaryngology (contact
Anna-Sophie Hoffmann ) and the Leibniz Institute of Virology.
Contact
Valentin Bärreiter , PhD Student
Ilka Grewe , Clinician Scientist
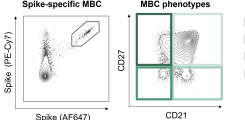
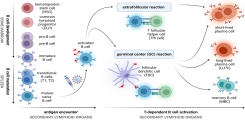
Dissecting humoral and B cell responses following vaccination
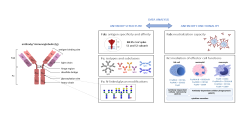
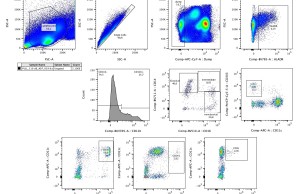
Upon vaccination, naive B cells specific for the vaccine antigen are activated and differentiate into different B cell populations. While plasmablasts and plasma cells secrete high levels of antibodies and are mostly short-lived, memory B cells can survive for years in the absence of the antigen and are important for long-term protection. Binding and neutralizing antibodies are considered important drivers of vaccine-induced protection against infection. In addition, non-neutralizing antibody functions such as antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) are increasingly recognized as an important part of antibody-mediated protection against disease, and are strongly influenced by the antibody subclass and N-linked Fc glycans.
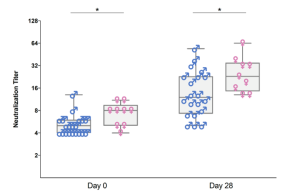
As part of different clinical studies evaluating vaccines against the human coronaviruses MERS-CoV and SARS-CoV-2, we are analyzing the induction and longitudinal dynamics of antigen-specific (memory) B cell responses. In addition, we are aiming at a comprehensive analysis of vaccine-induced antibodies using a systems serology approach. Besides the analysis of antibody isotypes, subclasses, ADCC and ADCP, we are collaborating with Prof. Sandra M. Blois (Glycoimmunology, UKE) to investigate the Fc glycan composition of vaccine-induced antibodies. To analyze the relations between different immune parameters and enhance the insight that can be gained from our multidimensional datasets, we are applying advanced statistical methods and computational tools in collaboration with Prof. Laura Richert (Statistics in systems biology and translational medicine, University of Bordeaux).
A comprehensive analysis of vaccine-induced B cells and antibodies is important to characterize the magnitude and quality of vaccine-induced immunity and to better understand the impact of different vaccine platforms and immunization schedules on the quality and persistence of immune responses.
Contact Marie Weskamm, Scientists
Publications
Weskamm LM, Dahlke C, Addo MM. Flow cytometric protocol to characterize human memory B cells directed against SARS-CoV-2 spike protein antigens. STAR Protoc. 2022 Dec 16;3(4):101902. doi: 10.1016/j.xpro.2022.101902. Epub 2022 Nov 15. PMID: 36595922; PMCID: PMC9663734. Weskamm et al
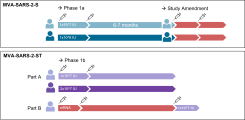
Weskamm LM, Fathi A, Raadsen MP, Mykytyn AZ, Koch T, Spohn M, Friedrich M; MVA-MERS-S Study Group, Haagmans BL, Becker S, Sutter G, Dahlke C, Addo MM. Persistence of MERS-CoV-spike-specific B cells and antibodies after late third immunization with the MVA-MERS-S vaccine. Cell Rep Med. 2022 Jul 19;3(7):100685. doi: 10.1016/j.xcrm.2022.100685. Weskamm et al
Fathi A, Dahlke C, Krähling V, Kupke A, Okba NMA, Raadsen MP, Heidepriem J, Müller MA, Paris G, Lassen S, Klüver M, Volz A, Koch T, Ly ML, Friedrich M, Fux R, Tscherne A, Kalodimou G, Schmiedel S, Corman VM, Hesterkamp T, Drosten C, Loeffler FF, Haagmans BL, Sutter G, Becker S, Addo MM. Increased neutralization and IgG epitope identification after MVA-MERS-S booster vaccination against Middle East respiratory syndrome. Nat Commun. 2022 Jul 19;13(1):4182. doi: 10.1038/s41467-022-31557-0. Fathi et al
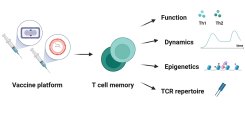
T cell memory after coronavirus vaccination
Memory T cells are an essential part of the adaptive immune system as they clear infected cells and are necessary for the induction of an efficient humoral response. Therefore, their induction is important to consider during vaccine development. Novel vaccine platforms such as mRNA and viral vectors have been shown to induce strong T cell immune responses, which differ depending on homologous/heterologous prime-boost strategy and time between vaccinations. In this project, we are analyzing the dynamics and function of the T cell response after vaccination with the licensed COVID-19 vaccines and MVA-based vaccine candidates. A better understanding of the post-vaccination T cell response will help to define correlates of vaccine-induced protection, especially against viral variants.
Contact Leonie Mayer, Scientist
Publications
Harrer CE*, Mayer L*, Fathi A, Lassen S, Ly ML, Zinser ME, Wolf T, Becker S, Sutter G, Dahlke C, Addo MM; MVA-MERS-S Study Group. Identification of a spike-specific CD8+ T cell epitope following vaccination against the Middle East respiratory syndrome coronavirus in humans. J Infect Dis. 2024 Jan 9:jiad612. doi: 10.1093/infdis/jiad612. Epub ahead of print. Harrer and Mayer et al
*authors contributed eqally
Molecular Virology of Emerging Viruses
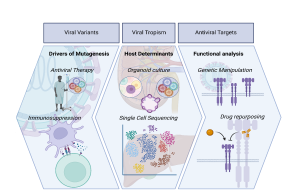
Viral Variants
Approximately 22% of the global population display at least one underlying condition, which puts them at higher risk of adverse outcomes for infectious diseases. Indeed, the recent SARS-CoV-2 pandemic highlighted the vulnerability of specific populations with co-morbidities towards less favourable disease outcomes. Amongst those, immunosuppressed patients have become the focus of attention. This group includes individuals with various malignancies and immunologic diseases, as well as patients who have undergone solid organ transplantation. The latter are typically treated with immunosuppressive drugs.
There is a consistent body of evidence reporting prolonged or chronic infections with SARS-CoV-2 in immunocompromised individuals. Certain reports even proposed that SARS-CoV-2 Variants of Concern (VOCs) which became globally dominant, including the Alpha variant, are thought to originate from individuals with compromised immune systems. This phenomenon has been observed in chronic infections with several viruses, such as DENV and hepatitis E virus (HEV). Continuous replication of the virus potentially favours the accumulation of mutations in order to adjust to environmental and immunological pressure and therefore presumably facilitates the emergence of new variants with enhanced epidemic potential. Additionally, immunocompromised patients often require pharmacological interventions to clear infections, which may also further drive viral mutagenesis. Direct acting antivirals such as the nucleotide analogue Remdesivir or Ribavirin (RBV) facilitate a so-called “error catastrophe” during viral replication, which leads to defective or replication-incompetent viral genomes and subsequent viral extinction. However, in some cases the selective pressure drives viral mutagenesis leading to the formation of intra-host populations.
The emergence of novel viral variants presents a risk to the general population by affecting various aspects such as transmission dynamics, public health measures, vaccine efficacy, antiviral treatment, disease severity, and pathogenesis, among others. Therefore, it is crucial to thoroughly investigate potential drivers of mutagenesis and understand the impact of specific mutations.
Here, we aimed to explore viral replication under immunosuppressive or antiviral therapy to better predict and manage the spread of variants, potentially leading to the development of new antiviral drugs and therapeutic strategies.
Viral Tropism
Viral tropism, the tendency of viruses to infect specific cell types or tissues within a host organism, is a fundamental aspect of viral pathogenesis. The ability of a virus to target and infect particular cells is determined by a complex interplay of viral and cellular factors. Some viruses exhibit a narrow tropism, infecting only a limited range of cell types, while others display a broader tropism, infecting multiple cell types throughout the body. Viral tropism can play a critical role in determining the outcome of infection. Understanding why certain viruses cause more severe disease in some individuals compared to others is crucial for developing effective treatments and preventive measures.
Therefore, we explore the intricate interactions between viral pathogens and their hosts, including host immune responses, viral genetic diversity, microenvironmental factors, and cellular susceptibility. By elucidating these factors, we aim to improve our understanding of viral pathogenesis and uncover the specific interactions between viral pathogens and host cells to identify novel drug targets and develop strategies to block viral entry or replication selectively.
Antiviral Targets
Viral infections pose a significant global threat, contributing to disease, mortality, and economic losses. This raises significant concerns, especially given the limited availability of antiviral drugs for treating viral disease. Recent viral outbreaks including Zika (ZIKV), Ebola (EBOV), and the more recent SARS-CoV-2 pandemic highlight the urgent need for novel antiviral strategies. Therefore, identifying and targeting specific molecular components of viral entry, replication, and assembly processes offer promising avenues for intervention. Targeting viral entry mechanisms, such as viral surface glycoproteins and host cell receptors, can block the initial stages of infection and limit viral spread within the host. Additionally, one key antiviral target is viral replication machinery, including enzymes essential for viral genome replication and protein synthesis. Inhibiting these enzymes can disrupt viral replication cycles, preventing the production of infectious viral progeny. Host factors essential for viral replication and propagation also serve as potential antiviral targets. By disrupting host-virus interactions or modulating host immune responses, we can inhibit viral replication and reduce disease severity.
We aim to find agents that may exploit conserved viral structures or host factors shared among different viral families, providing a versatile approach to combating diverse viral infections.
Contact
Toni L. Meister , DZIF Junior Group Leader
Dissecting Sex Differences in the Immune Response to Vaccines
Vaccines are one of the most impactful public health interventions, preventing millions of infections and deaths worldwide each year. As illustrated by recent epidemic or pandemic outbreaks caused by emerging viruses such as Ebola virus, MERS-CoV and more recently SARS-CoV-2, the need for rapid and strategic vaccine development remains paramount.Men and women differ in their immune responses to vaccination, with women typically developing higher antibody responses but also experiencing more adverse reactions following vaccination than men. However, the exact mechanisms and pathways involved in the differential vaccine outcomes between the sexes remain incompletely understood.
We propose to prospectively investigate and dissect sex-specificity in vaccine immunity against two important emerging respiratory pathogens with pandemic potential and high global significance: MERS-CoV and SARS-CoV-2, two recently discovered novel coronaviruses. A detailed understanding of the molecular factors associated with sex differences in vaccine-induced immune responses may ultimately allow strategic modulation of vaccine immunity and promote individualized vaccine design.
This project is part of the DFG-funded Research Unit 5068 on "Sex differences in immunity".
Contact Tamara Zoran, Scientist
Publications
Find out more using the DFG MenschMikrobe App ( Download Flyer)
Fathi A, Addo MM, Dahlke C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front Immunol. 2021 Jan 8;11:601170. doi: 10.3389/fimmu.2020.601170. Fathi et al
Evaluation of sex differences in immune responses after immunization against COVID-19
This project evaluates early innate immune responses following immunization with the vaccine candidate MVA-SARS-2-S compared to immunization with an approved mRNA vaccine against COVID-19. At longitudinal time points after each immunization, cytokine levels are analyzed by Luminex, diverse innate cell populations are assessed using flow cytometry, and RNA expression of innate cell subsets are measured using RNASeq. The aim of the project is to evaluate whether there are differences in the vaccine-induced innate immunity between male and female study participants.
This project is funded by the German Center for Infection Research (DZIF)
Contact Anahita Fathi, Clinician Scientist